



Emission Reduction and Treatment Options
General
The PCA and the EPA have calculated the potential amount of equipment required by US cement producers to meet proposed regulations. There is significant variation in the estimates. If similar strictures were placed on producers in other countries similar equipment would be required. Since the US represents less than 5% of installed capacity, the potential for APC equipment could be staggering. (US would require 125-140 wet scrubbers, 120-143 ACI with baghouse, 15-100 RTO systems.)
Because many US plants are older, space constraints may add to the obstacles facing those operators attempting to install equipment or modify process in order to meet new regulations.
It is generally believed that to meet EPA regulations cement manufacturers will have to implement a holistic approach concurrently using a variety of techniques. The approaches to reducing emitted pollutants include:
Reduction of Pollutants Entering Process
Use raw materials with fewer pollutants
Use fuel with fewer pollutants
Remove pollutants from raw materials and/or fuel prior to use
Adjust process to avoid producing, remove or capture pollutants during processing
Adjust process to produce pollutants in a form which can be more readily captured.
End of Pipe Emission Control Strategies
Capture pollutants after the process and prior to exhaust gas or waste enters the stack or waste stream.
|
Emission Control Strategies |
Reduction of Pollutants Entering Process
Fuel Selection
Plants may consider utilizing a fuel, such as natural gas, in order to reduce the pollutants entering the process. Many plants are located in areas where natural gas may not be available. Additionally alternate fuels may be more costly than the present fuel mix. (Many plants use a mixture of fuels including coal and waste such as old tires or sewage products.)
The use of alternate "green" fuels represents a primary opportunity for reducing CO2 emissions. Waste derived fuel can be used to replace some or all of the fuel. CO2 is produced as a result of the combustion of waste derived fuel.
Fuel Cleaning
Fuel cleaning, including coal cleaning, is a potential method for removing pollutants prior to the manufacturing process.
Raw Material Pre-Processing
Mercury (and other pollutants) may be removed from the raw materials prior to the manufacturing process. Environmental Quality Management has a patented process for desorbing mercury from raw materials.
Dry Process Plant Design
Most new cement manufacturing facilities are of the dry process design. The flue gas is re-circulated back to the raw mill using a pre-calcinator arrangement. The kiln gas is used to sweep the mill of finely ground raw feed particles. The temperature in the raw mill is typically between 90 and 120°C. In this manner the raw mill operates as the primary cooling system. A cooling tower is the secondary cooling system and serves as the sole cooling method when the raw mill is off. Since the flue gas is circulated through the raw mill, the raw materials adsorb components in the gas (almost as a scrubber.) The material (including the adsorbed Hg) from the raw mill is then stored in silos until it is needed in the process. Most models and operations have found substantial differences in emissions depending on whether the mill is off or on. HCl, Hg, THC and dioxin emissions can increase by orders of magnitude when the mill is off.
The affect on emissions of the materials in the raw mill is particularly noticeable with Hg. The EERC did a study of Hg emissions in a variety of kiln arrangements. In the 4kilns with precalcinators and preheaters that they studied they found Hg levels when the raw mill was off to far exceed the levels when the raw mill was on. The data they collected was as follows:
| Kiln Number | Raw Mill On Hg µg/dscm | Raw Mill Off Hg µg/dscm | Magnitude Difference |
| 1 | 2.52 | 87.24 | 34.6 times |
| 2 | 237 | 2800 | 11.8 times |
| 3 | .28 | 11.37 | 40.6 times |
| 4 | 16.62 | 793.65 | 47.7 times |
When the raw mill is on Hg can be absorbed and released in an equilibrium-like balance. Some experts have suggested that when the raw mill is off emissions can be partially controlled (50-70%) using a bleed system (see Fabric Filter info below for more information.) One possible reason for the variation in Hg emission levels when the kiln is off is that the Hg levels vary significantly over time peaking as much as 10 to 14 hours after the raw mill is taken off line.
Al Linero of the Florida Department of Environmental Protection provided the following information about new projects:
|
Several new kilns started up in Florida that are subject to the present EPA mercury (Hg) rule for new cement kilns (that is under reconsideration.) The allowable emissions are 41 micrograms per dry standard cubic meter (ug/dscm) at 7 percent oxygen when the raw mill is on and ALSO 41 ug/dscm when the raw mill is off.
One
kiln conducted the required stack tests and had the following results: The limestone is low in Hg but is relatively high in chloride that suggests possible oxidation. At the same time, a new kiln in Texas tested and reported low Hg (<< 41 ug/dscm) with the raw mill on and with the raw mill off. The Texas kiln sheds light on the benefits of low Hg in the raw materials and additives. The Florida kiln sheds light on the Hg in additives like fly ash. But it also suggests the role of chlorides and temperature in concentrating and enriching the raw meal and particulate control equipment dust with enough Hg to make it feasible to remove some Hg by a raw meal or dust bleed. It may be possible to mimic the oxidizing action that must be occuring at the Florida kiln by an SCR system or some oxiding agent in the conditioning tower (effectively a scrubber) or doping carbon or more concrete friendly sorbent with some amount of chloride (within tolerable levels) and using the dust in masonry cement.
|
PCA (Portland Cement Association) contracted a study titled "Inherent Mercury Contols Within the Portland Cement System" detailing Hg characteristics and emissions related to different kiln configurations. They determined that it is possible that Hg may form a complex mercury silicate which may be incorporated within the clinker material if the mercury silicates are thermodynamically stable at the temperature of the clinker exiting the kiln.
It seems likely that in a typical modern preheater/precalcinator kiln the gas moving through the precalcinator/preheater section will be hot enough to volatize the Hg and the volatized Hg will be carried by the gas away from the kiln. Clinker sample Hg levels are typically below detectable levels.
Mercury Speciation
Changes in or additions to the process including combustion additives such as Chlorine salts (NaCl or CaCl2) may help to change the speciation of mercury to forms that are more readily collected by "end-of-pipe" controls.
Oxidizers (including SCR, ozone or UV processes) have been proposed (but not proven) to reduce emissions from the process.
Pyro Optimization
Plant designers and operators have focused on optimization of the heating process (ie burner design etc) as this reduces the cost of fuel. Some estimates are that the typical pyro heating process is only 34% efficient. Burners have been re-designed to optimize heating. In addition to optimizing the actual burning process, the heat is reused for preheating raw materials and re-circulated to the kiln. (Wet process systems require more fuel and thus produce more CO2.)
Alternative Raw Materials
Fly Ash
CCB (coal combustion byproducts) such as fly ash (primarily produced in the process of burning fuel for power generation) is a waste product of the utility industry. Fly ash can be used as an admixture (to improve durability and economy) in the production of cement or in the production of clinker (which is one of the raw materials used in the production of cement.)
Any carbon contained in fly ash used as raw material would be re-volatized in the kiln. Therefore, although the use of fly ash may reduce net CO2 emissions, the introduction of additional mercury with the ash may lead producers to avoid the inclusion of ash in the process.
|
In 2005 21% of fly ash was used in concrete (not including the material that was incorporated in the raw feed.) The US EPA set a goal to increase the use of CCBs as SCM (supplementary cementitious material) in concrete by 50% from 12.4M tons in 2001 to 18.6M tons in 2011 with the hope that the material would reduce the GHG (green house gas) emissions of cement production by a corresponding 5M tons. Concurrently, in the US, environmental regulations affecting utility plants have resulted in increased levels of carbon in flyash. Low NOx burners used to reduce NOx emissions result in an increased amount of residual unburned carbon. Additionally, regulations requiring the capture of mercury and other components have resulted in the injection of carbon into the exhaust gas by many utilities to capture gas phase mercury. Unless the utility installs fabric filters both before and after the carbon is injected, the carbon (and the captured mercury) ends up in the fly ash which the utilities hope to sell to cement producers. |
Studies have shown that carbon in the fly-ash makes it unsuitable for the
production of cement.
Air voids are needed in cement or concrete that is exposed to freeze and thaw cycles. Concrete needs approximately 6% air entrainment (by volume.) AEAs (air entrainment agents) are added to most concrete product. AEAs can be sorbed onto both unburned carbon and activated carbon making the AEAs unavailable to facilitate the incorporation of air bubbles required . The EERC (and others) have performed testing which determined that even small amounts of activated carbon in fly ash can have a drastic effect on the amount of AEAs required for cement production.
However, a study published for the Ash Utilizaton Symposium (2003) titled, "Commercial Demonstration of High Carbon Fly-Ash Technology in Cement Manufacturing" determined that the fly-ash could be used as a raw material for the production of clinker. The carbon improves the efficiency of the pyro-process and affects the crystalline structure of the clinker material in a manner that has a positive affect on the resultant cement. (The mercury that is added to the process with the fly-ash and carbon increases the mercury emission levels of the cement manufacturing process.)
ACI Benzine Bromine Injection CFBA C-S-H Dust Shuttling and Wasting ESP FF FGD Scrubber RTO Sodium Injection SCR Trona
Injection Basics
The injection of any material with the expectation that the injected material will either react with or adsorb a pollutant (or other material) is a popular and successful pollution control strategy. There are several factors which affect the efficiency of this sort of process.
![]()




![]()

![]()
![]()
The injected material must either contact or be in close proximity to the material to be controlled. Increased control efficiency can be accomplished by either increasing the amount of material injected or improving the contact of the materials: either by better injection of the material or a longer amount of time for the materials to react with each other (residence time.) Temperature is frequently crucial to collection efficiency.
There may be a trade-off between the cost of improving the injection (or mixing) of the materials and the cost of the materials themselves. For example, if the sorbent is inexpensive it might be less expensive to inject more sorbent than to increase the number of injection locations. The size of the particles being injected is a factor since in most instances increased surface area equates with increased removal.
The residence time is the amount of time the chemicals are in close enough proximity to react. One effective manner to improve removal efficiency is the use of injected material prior to a baghouse. The injected material reacts with the gas in the ducting but continues to react as it collects on the filter material. The dust cake serves as a secondary reactor location. (Some data has suggested that in some instances the use of membrane filtration media (which is more thoroughly cleaned) reduces the collection of pollutants dependant on residence time of injected materials for reaction or adsorption.)
Activated Carbon Injection (ACI)
ACI is the EPA proposed technology for the control of Hg ( in concert with wet scrubbing.)
ACI is the EPA proposed technology for the control of THC (with RTO as needed.)
ACI may be used for both mercury and THC removal, however the type of carbon used may be different in some instances. Although some experts have contended that It is possible that 2 ACI systems would be required (1 for Hg and 1 for THC,) carbon suppliers, including ADA, have stated that would not be the case.
Although the EPA had hypothesized that ACI would be able to capture approximately 90% of organic pollutants information collected during the regulatory comment period (ie since mid 2009) indicates that a 50% removal rate might be more feasible.
Typically used for mercury removal. When activated carbon is injected the mercury and spent sorbent (carbon) is typically collected with the fly ash in a particulate collection device (typically FF.)
Materials collected in the initial particulate collection device are typically re-circulated to the kiln. If activated carbon were injected prior to the initial baghouse the dust could not be re-circulated. if it were, the carbon would be released and the mercury would be re-volitized (and would have to be recollected endlessly.) To avoid contamination of the dust an initial particulate device may be used to collect the dust and a secondary (polishing) FF used to collect any remaining ash, carbon and mercury.
Activated carbon works better at lower temperatures. (Spontaneous combustion of activated carbon materials in a Toxicon (ACI/ polishing baghouse) system should be researched to assure appropriate safety issues are addressed in system design.)
An ACI system with a polishing baghouse may cost 10 to 15 times as much as an ACI system alone. Therefore, it might be practical to use ACI in front of the primary FF and forego the re-circulating of the dust to the process. FF dust could possibly be shuttled to the cement mixing area and incorporated in the product to the extent that the product will continue to meet specs. As noted above, a limited amount of carbon in flyash may improve cement product characteristics.
ACI increases ooperating costs. The activated carbon can cost in excess of $2000 per ton of Hg captured (increasing the clinker cost by as much as $.60 per ton.
ACI has not been sufficiently tested to determine if it will be sufficient to remove Hg alone or if a RTO would be required in addition to ACI. Studies indicate that elemental Hg is intially captured but then released by activated carbon. Therefore, the speciation of the Hg significantly affects success of removal.
Activated Carbon can be chemically impregnated with other materials (notably bromine or sulfur) to improve efficiency. The system efficiency is affected by temperature, residence time and type of carbon.
Benzine can be used for control of THC in lieu of RTO.
Halogens, such as bromine, affect the oxidation of mercury. The speciation of the mercury (ie how much of it is elemental vs different oxides etc) substantially affects the ability of control devices to capture Hg. Elemental Hg has been shown to be more difficult to capture. Bromine may be added to increase oxidation levels.
Bromine can be added alone or in the form of brominated carbon. Although the cost of bromine alone may be lower, the handling and generation of bromine adds some issues for cement plant operators. Bromine is highly toxic, hard to work with and must be generated on site. bromine alone will not result in sufficient Hg capture without some activated carbon.
Bromine may be used in the scrubber for HG removal. If so, the bromine and Hg will appear in the scrubber waste.
Calcium Silicate Hydrate (C-S-H)
This material is essentially ground up cement and has been shown in some studies to function as well as activated carbon for Hg control
Circulating Fluidized Bed Absorber (CFBA)
A semi-dry scrubber utilizing injected lime, ash and water (and other sorbents as needed.) The particulate in the flue gas acts as the reactor. The particulate matter exiting the CFBA with the flue gas is captured in a cyclone and recirculated to the system (byproducts can be returned to the product if no problematic sorbents (like Carbon) are injected.) (FLSmidth produces a CFBA which they market to the incinerator industry called a Gas Suspension Adsorber.)
This system can/should have a high level of sorbent control; usage depending on the status of the raw mill. (When the mill is off sorbent is added to avoid the build-up of pollutants.)
Temperature is somewhat sensitive. Typical range is 100-120C to remain above the dew point but remain within optimal absorption range of the materials
Theoretical capture rates: (SOx 95-98%, PM and condensibles 50-90%, Hg 50-95%, HCl 95-98% THC 50-90%)
Electrostatic Precipitator (ESP)
Removal of solid matter using electrical charges.
FF is the EPA proposed technology for the control of particulate (PM.)
It is important to understand that lacking additional controls, reducing the temperature at the inlet to the collector may improve Hg capture since the Hg compounds most frequently present condense below 270°C. (Because the vapor pressure of most of these compounds is high they tend to be relatively volatile (ie some or all of the material changes to a gaseous state even below the temperature at which they would normally condense when the pressure increases as it does inside a closed system or container such as a baghouse.)
A fabric filter or baghouse uses filtration material to remove solid materials. The materials cannot pass through the filter and are captured. Materials in a gaseous phase would not be readily captured by a fabric filter.
However, a dust cake builds up on the filter. The dust cake is actually part of the filtering process because the gas moves through both the dust cake and the fabric. (Any solid state or adsorbed chemicals which have been introduced into the process for emission control are captured by the filter and become part of the dust cake. Because the gas must pass through the dust cake and the filter, the dust cake provides a secondary opportunity for materials which have been introduced into the system for emission control to react with the exhaust gas. For instance, if activated carbon is injected into the ducting it reacts with the gas in the duct and then collects on the filter as part of the dust cake and reacts with the gas passing through the dust cake.) Fabric Filter systems are self-cleaning. During the cleaning cycle the dust cake is removed (and thus any filtering provided by the dust cake varies depending on how clean the filters are.)
Oxidized Hg may be more easily captured in a FF than elemental Hg. Oxidizing agents can be introduced into the process or gas to facilitate the oxidation of Hg.
Particulate (dust) collected in a FF may be re-circulated back to the process, used in the final cement product or disposed. It is typical that some of the cement kiln dust (CKD) is removed (bled) from the system to lower the alkali salts in the clinker. This is called dust wasting and may be required to assure that the clinker meets specifications. Some portion of the mercury (and other materials which build up over time as the CKD is re-circulated) are removed in this manner. There has been some speculation that dust wasting could be an effective Hg control method. Titan America did a detailed analysis of this possibility with their materials management program and presented their results at the 2010 IEEE ISA/PCA meeting.
| Daniel Crowley of Titan America Medley Florida presented a paper titled “Cement Kiln Mercury Reduction Strategies A Case Study in Materials Management” at the 2010 IEEE Cement Industry Conference. He provided insight into Titan’s experience with Hg monitoring and control strategies. Titan’s experience indicates that to meet EPA proposed limits using continuous dust wasting they would have to waste 10% of all feed produced. By wasting dust only with the raw mill off (after Hg levels had peaked which may be 12-14 hours after the raw mill goes offline) Titan found that they could achieve a 43% reduction in Hg levels; concluding that dust wasting will probably not be a practical means to meet EPA proposed Hg limits. |
It has been proposed that Hg be removed from the dust which might otherwise be wasted. In this manner the volume of material and gas to be treated would be a fraction of the total exhaust. This could be achieved via ACI and a polishing FF which would be significantly smaller than the main baghouse or by a system which heats the dust in the internal loop between the main baghouse and the kiln to re-volatize the and remove the Hg.
| Peter Paone, Manager of Pyroprocessing at FLSmidth presented a paper titled "Mercury Controls for the Cement Industry" at the 2010 IEEE Cement Industry Conference. He provided insight into FL Smidth's patent pending Mercury Roasting system and alternative methods of Hg removal. The Mercury Roasting system, which has been pilot tested but not yet installed in a full plant application, uses hot gas (typically from a kiln gas bypass or cooler take-off) to re-volatize the Hg in the dust in the internal loop between the main baghouse and the kiln. The Hg is captured in a Gas Suspension Absorber system. (The dust is returned to the system and the sorbent and Hg is either wasted or used as a cement additive in the finish milling. The dust is separated in an ESP and the sorbent is collected in a baghouse. |
Filter dust may be introduced into the cement product (to the extent that the cement product meets specs.) When filter dust from clinker production is incorporated in cement product it must be moved to the cement production area therefore this is sometimes referred to as dust shuttling.
FGD Scrubber (Flue Gas Desulphurizaton)
Scrubber with limestone material is the EPA proposed technology for the control of HCl.
It should be noted that in the typical preheater/precalcinator cement plant the raw mill functions as a scrubber. That is why the emission levels with the raw mill offline are higher than when the mill is online.
Scrubbers may be either wet or dry designs. They use calcium-containing material for SO2 control. Although FGD systems are typically installed for sulfur removal, gaseous oxidized mercury is also collected in wet FGD systems. A study titled "Fate of Mercury Collected from Air Pollution Control Devices" determined that between 77-95% of gaseous mercury is removed and "largely ended up in the scrubber solids." According to the report the mercury appears to be concentrated in the (predominantly iron oxyhydroxide) fine particles and not associated with the solid calcium. The mercury can end up in the gypsum fines and the FGD byproduct. There are some indications that scrubbers are not successful in removing elemental Hg.
Wet scrubbing is generally not considered to be practical in cement plant operations due to economic considerations including the costs associated with the water and waste water processing (being useful only during raw mill off periods.)
Synthetic gypsum produced from dewatered FGD byproducts may be usable as a raw material for cement. (Cement is a combination of milled clinker gypsum and additives if/as needed.) Currently many cement manufacturers obtain synthetic gypsum from utility scrubber applications.) Currently the EPA has not established a maximum amount of Hg allowed in the synthetic gypsum.
Regenerative Thermal Oxidizers (RTO)
RTO systems use natural gas flame to combust THC. Units currently on the market have performance limits of 5-10 ppm. Plants may not have a local source of natural gas, and the performance limits of available technology may not be sufficient to meet proposed regulations.
SCR (Selective Catalytic Reduction)
Ammonia is injected into the flue gas in front of an SCR to convert NOx to Nitrogen and water.
Both SCR and SNCR can create condensibles and increase PM2.5 and HCl. Systems are prone to problematic ammonia slips.
Sodium Bicarbonate (baking soda) or Trona can be added either to the feed materials or injected (either in a dry or liquid form) to the process materials or flue gasses to control SO2, SO3 and HCl. Sodium also has an indirect affect of improving the Hg absorption capacity of fly ash or carbon.
| In addition to pollutant removal, sodium sorbents may serve a process related function when used in instances where high Sulfur content causes overheating in the chain section, ring formation in the preheater or kiln, or tower plugs. When sulfur content is high plant disturbances and interruptions may be caused by the build-up of hard solid material. The reacted material formed when the sulfur reacts with sodium sorbents does not affect process operation. |
Sodium Bicarbonate or Trona reacts to produce Sodium Chloride or Sodium Sulfate (both are dry ie. no liquid is produced in the reaction.) The absorbent efficiency is much better at higher temperatures. (275°F is the minimum acceptable temperature but application temperatures of up to 1500°F are acceptable. Therefore the sorbent can be inserted into the process essentially anywhere after the kiln.)
Sodium Bicarbonate and/or Trona is very reactive with HCl and is a popular method for reducing SO2 and HCl in cement kilns in the EU. Although sodium sorbents can be introduced using a semi-wet or spray dryer scrubbing arrangement, a simple dry injection system using a blower, storage (either a silo or unloader and supersack arrangement) and lances usually provides sorbent in a manner sufficient for cement kiln applications. If removal is insufficient additional injection points can be added. Solvay reports having supplied sorbent to more than 1000 kilns in the EU. SO2 can be reduced by 80 to 90% and HCl can be reduced to less than 6ppm with a relatively small (.5 to 2.0 NSR (normalized stoichiometric ratio)) amount of injected material.
Removal efficiencies are better when used upstream of a baghouse than when an ESP is used for particulate collection. Although the minimum reaction time is approximately 1 second, the cake on the filter material provides increased opportunity for reaction and removal.
Hg removal is facilitated by the injection of sodium sorbents even though there is no reaction or adsorption between the materials. SO3 can interfere with the adsorption of Hg by either fly ash or activated carbon. Typical SO3 levels in cement kiln applications range from 1-10ppm. The reduction of SO3 levels improves Hg removal efficiencies. SO3 concentrations as low as 3 or 4 ppm can dramatically reduce Hg adsorption.
Trona is a rock (ore) mined principally in the Unites States (Wyoming) and shipped as a powder. Trona is very porous (particularly at high temperature) with a large surface area (key to successful reaction.) Trona injection has been found to remove (via a reaction process) some heavy metals, particularly selenium from the flue gasses. (If a wet scrubber were to be included in the emission control strategy, the removal of selenium prior to wet scrubbing could reduce costs of waste water treatment. Systems to remove selenium from utility plant scrubber waste water can cost as much as $20M.)
Mercury Emission Control Strategies
Emission Control Strategies Following Initial Baghouse Collection
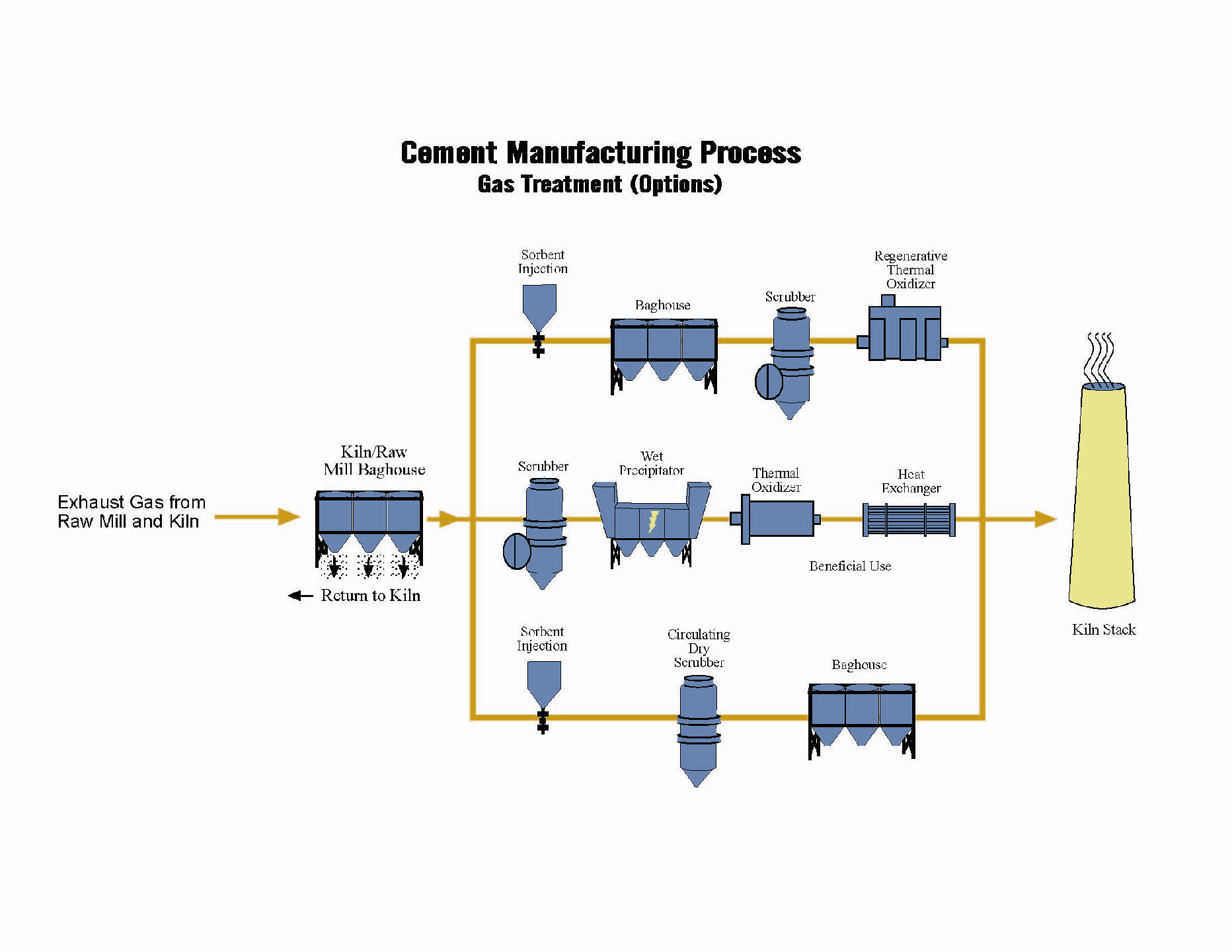
Typically, the first step in all treatment configurations is a baghouse utilized for reduction of particulate, consisting primarily of the filter bags, a bag cleaning device, and dust handling equipment. The filter dust from the baghouse can be returned to the kiln to avoid disposal costs. The next steps in treatment depend on several key factors. The first factor is whether the installation is a retrofit to an existing kiln or a new facility. The second factor relates more to the clean air permit requirements of the site.
With the proposed new mercury rules (US) the first particulate collector will be followed by a second baghouse to collect mercury. Activated carbon will be injected ahead of the bags
The following points contain a description of the three primary post-baghouse treatment technologies and the constituents that they treat:
1. Valveless regenerative thermal oxidizer (VRTO) This technology thermally treats the CO and VOCs by heating them to 1,600degreesF. Internal ceramic heat exchangers ensure that only minimal amounts of additional fuel are required. Unfortunately, the heat that is required to treat the CO and VOCs has an unfavorable reaction on the SO2 and SO3. At elevated temperatures, an equilibrium occurs between these two constituents where, depending on oxygen levels and several other parameters, a portion of the SO2 can convert into SO3, which is far more difficult to treat. The equilibrium tendency to create more SO3 can be reduced by optimizing the system combustion temperature and retention time. The following features of a VRTO will have advantages in cement manufacturing applications:
* Constantly moving rotary distributor to reverse the exhaust flow in the heat-exchanger beds. This enables avoidance of deposits on valves that can cause seal leakage;
* Proper and controllable retention time;
* A heat exchange media that is cleanable and provisions for periodic washing of the media; and
* Proper material selection to avoid corrosion.
2. Wet flue gas desulfurization scrubber (FGD) In this process, limestone is used as a reagent to absorb the SO2 and SO3 from the exhaust stream. The SO2 combines with the limestone (CaCO3) to form CaSO3 and is further oxidized to CaSO4 by a forced oxidation through air injection. SO2 levels are reduced by 96% to 98%, and SO3 is reduced by 30% to 90%. This wide range for the SO3 reduction is due to the fact that aerosols are formed that are very difficult to remove. To achieve the higher values, baffles or trays must be used to force a turbulent reaction with the limestone slurry reagent. Depending on the sequence of technologies selected, high SO3 removal efficiencies may or may not be needed in the scrubber. The resultant byproduct of this scrubbing process is gypsum (CaSO4). If properly dewatered and if the CaSO3 level in the gypsum is kept low, 100% of the gypsum can be reused and returned to the manufacturing process.
The scrubber process equipment consists of a limestone slurry preparation system, spray adsorber with quench zone, and mist eliminator for the exhaust stream. Other components include a combined recycling and forced oxidation tank to produce the gypsum, as well as gypsum dewatering, gypsum handling, and water recycling equipment. The entire system is waste-water free, but it is important to look at the chloride content in the make-up water as well as the limestone to know what the equilibrium chloride concentration will be in the scrubber. This chloride concentration is a major factor in scrubber material selection.
3. Wet electrostatic precipitator (ESP) The wet ESP can be mounted directly on top of the scrubber tower in order to minimize the amount of duct and ground space. With this configuration, all liquids will run into the scrubber and will be neutralized by the pH-controlled slurry in the scrubber. The wet ESP exposes the aerosols and particulate to a negative electric field through the use of discharge electrodes. These particles are then attracted by the collector tube and trapped in a water film within the tube. The water film ensures that particles and aerosols are not re-entrained after capture. This water film also has the function of diluting the acid and maintaining a clean collector surface.
The ESP should be used in applications where very low emission values are required. Although expensive, it is the best device for removing the difficult to control SO3, as well as submicron particulate. Note that if a scrubber and ESP are coupled together, the scrubber can be configured with lower SO3 removal and lower pressure drop since the ESP will eliminate virtually all of the SO3.
Technology combinations There are three main options for combining these technologies:
OPTION 1-Kiln/Baghouse/FGD/Wet Stack- This combination will have the least frequent use since it does not treat VOCs or CO. In some locations, VOCs and CO are not heavily regulated and therefore this combination is possible. Depending on the type of scrubber used, the effect of this treatment will be 96% to 98% SO2 removal and 30% to 90% SO3 removal with very little effect on the other emission compounds.
OPTION 2-KIln/Baghouse/FGD/VRTO/Dry Stack-This configuration is more common. Installing the FGD system prior to the VRTO reduces the amount of particulate entering the unit and reduces the likelihood of heat exchanger blockage. Although the amount of particulate reduction primarily depends on the baghouse, it should be noted that even in this configuration, some moisture will pass through the scrubber mist eliminators carrying particulate that will deposit on the VRTO heat exchanger. An advantage of the Option 2 arrangement is that the amount of SO2 is reduced prior to the VRTO. Therefore, the equilibrium effect in the VRTO that drives SO2 to SO3 is minimized since most of the SO2 has already been removed. A disadvantage of this arrangement is that the exhaust enters the VRTO fully saturated with moisture, meaning that as it is heated, it passes through the temperature range with the highest corrosion potential (above water dewpoint and below acid dewpoint). Thus, very costly nickel alloys must be used at certain areas within the VRTO. Another disadvantage of this arrangement is a costly trade-off. If the VRTO heat exchanger is designed to achieve high thermal efficiency (low gas consumption), the outlet temperature will be below the acid dewpoint. Henceforth, all outlet duct and the stack must be resistant to acid condensation and its high levels of corrosion. If the outlet temperatures are raised above the critical acid dewpoint temperature, then the VRTO will consume very large quantities of natural gas. Therefore, the choice is either high energy costs or high material (alloy) costs.
OPTION 3-Kiln/Baghouse/VRTO/FGD (with optional ESP)/Wet Stack-The advantages of this arrangement are said to be that the VRTO can be sized to the highest energy efficiency without concern for corrosion since the exhaust enters the VRTO already above the acid dewpoint. The second advantage is said to be that the exhaust never is in the high corrosion temperature zone, therefore allowing a lower cost material selection in many areas of the system. As well, an ESP can be added in the base system supply or on top of the scrubber tower if deemed necessary.
The disadvantages of this arrangement are that the VRTO will see a potentially larger amount of particulate in the event that a baghouse bag breaks. This, however, can be handled through an automatic exchanger bed washing system or preventative controls that isolate the broken bag. Another disadvantage is that the total amount of SO2 will now enter the VRTO resulting in a higher equilibrium conversion to SO3. If the scrubber is properly designed, this higher SO3 output can be handled usually without the ESP.
There are several other factors that can affect the system's performance, such as the amount of organic sulfur in the exhaust. The VRTO will convert the organic sulfur to SO2, and therefore, depending on the exact quantities in the exhaust, there can be additional trade-offs between the options listed above. If, for example, there is a significant amount of organic sulfur, then Option 3 is preferred since the SO2 will be removed in the scrubber.
It is clear from the many variables in cement manufacturing process that each application must be reviewed to determine the best combination of technologies. When analyzed properly, the solution should optimize corrosion prevention, emissions reductions, and the low cost of ownership so that long-term emissions compliance can be economically realized.
SNCR Injection for control of NOx
SNCR (Selective Non-Catalytic Reduction) can be used for the control of NOx. The use of SNCR is widespread in Europe, principally on PH or PHC kilns. (Use on long wet kilns is still somewhat experimental.)
NOx affects haze and thus is regulated in the US under the Clean Air Act, the Clean Skies Legislation, the clean Air Interstate Rule (CAIR,) Ozone Transport Commission.
The reaction is as follows: 4NO + 4NH3 + O2 yields 4N2 + 6H20
Reagents: Possible reagents include Ammonia (NH3) or other Nitrogen containing agents (including Urea, Cyanuric Acid, Biosolids, Processed Photographic water.) The reagent is injected into the hot kiln gas. There are several possible injection locations depending on the type of kiln (process):
PH/PC
between the calcinator ant the 4rth stage cyclone.
between tertiary air and 4rth stage cyclone.
between top of preheater and 4rth sage cyclone
calcinator
bottom of calcinator
kiln feed shelf before calcinator
betwee bottom preheater stage and calcinator
calcinator before lowest stage cyclone
Preheater
kiln inlet (lowest part of preheater)
top of calcinator and crossover duct
Long wet kiln
kiln near feed end
Reaction is temperature sensitive with a range of 850 to 1050°C
NOx reduction of as much as 90% has been experienced (however NOx reductions in a recent study done for the Portland Cement Association ranged from 15 to 90%.)
Injection of ammonia may affect the synthetic gypsum from the scrubber
If Urea is injected water treatment system and heat traced storage and piping are required.